Do Your Employees Follow Protocol for Hospital Waste Disposal?
Dealing with hospital generated waste is not only the responsibility of the hospital administration but also of every hospital employee. Do you know if your employees are adequately trained on medical waste protocol?
Managing this waste should begin at the site of generation where waste should be properly collected and segregated from other non-hazardous waste in specific color-coded receptacles. Moving the hazardous medical waste through the hospital should be well mapped and identified by special carts. Hospital staff should be thoroughly trained on all waste handling procedures.
As a professional who works in healthcare, you’re faced with myriad stresses on a daily basis. In addition to ensuring proper patient care, following the right medical waste disposal procedures is key to the safety of everyone involved and not something you should have to worry about.
Here are just a few things to be aware of:
What is Hospital Waste Disposal?
It shouldn’t be confusing, but it can be. Understanding hospital waste disposal can be tricky, especially when you have staff members with varying levels of experience and training. Some items may not even appear used or soiled, but are possibly contaminated and dangerous. Your hospital staff must understand the nature of medical waste and proper handling and disposal protocols for the safety of everyone involved.
Avoiding Accidental Injuries
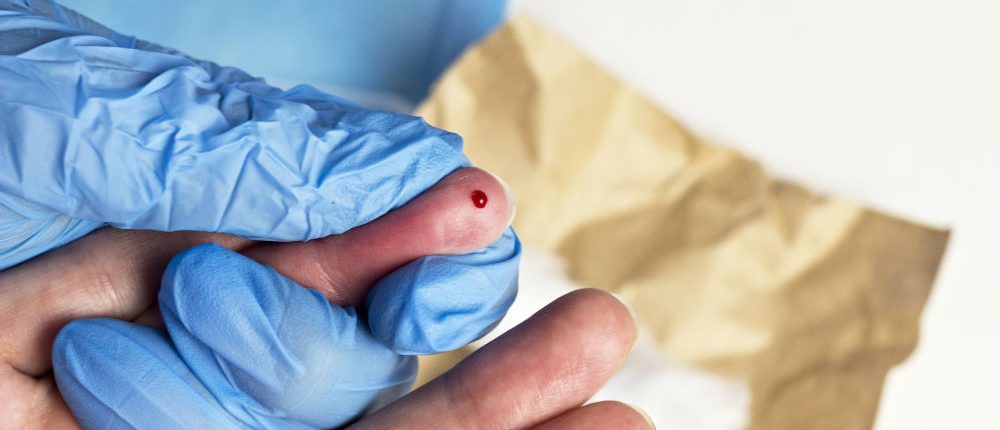
Sharps are often the first thing people think of when they think of medical waste and rightly so. The potential for injury with sharps is substantial and worth the concern. Disposing of sharps is definitely something your staff needs to be cognizant of to avoid potential injuries and accidents that can lead to the spread of illness. Using correct sharps containers is just one part of proper disposal. It’s crucial that everyone understands exactly how to handle them, how often to have containers replaced and what to do if they are stuck by a needle or other sharp.

Control For Overflow
So your people know their stuff on how and when to dispose of waste items, but wait, there’s more! What about the pickup and removal schedule? Without regularly scheduled and on demand pick-up, medical waste can fill up and overflow resulting in a messy and hazardous situation. Your facility needs a set pickup schedule based on the rate which waste is generated. Staff should also monitor the bins and note if they are filling at a faster rate so that on demand pickups can be scheduled.
Medical Waste Container Handling
While you could have your employees move, unlock and transport medical waste bins, why assume the liability? Medical Waste disposal companies are specially trained, equipped and insured to safely and properly transport and dispose of medical waste. There’s no need to risk mishaps, including spillage, could occur which endangers your team and your patients.
Who Will Be Your Trusted Provider
Just like in any other industry, there are those companies who claim to be experts but don’t have the proper certification and licensing to provide medical waste disposal. Choosing the wrong company to handle your medical waste can be hazardous and cause illness among your workers and your patients or clients. Be sure that you select an organization that is licensed and is OSHA, HIPAA and Health Department compliant.
Before health care staff interact with regulated medical waste, such as soiled items, needles and other sharp instruments, it’s important for them to receive training about the risks associated with medical waste and strategies for safely handling and disposing of it. Not only is this the right thing to do to ensure a safer, more healthful working environment, but it is also required by several states and regulatory bodies.

Know Your Hospital Waste Disposal Regulations
State Regulations
Regulations vary from state to state. While some require both orientation and annual refresher training, others don’t identify specific medical waste disposal training requirements. It is important to understand your state’s requirements and to make sure you have the appropriate training in place.
OSHA Guidelines
In addition to your state requirements, the Occupational Safety and Health Administration (OSHA) requires orientation and annual refresher training for staff members who deal with sharps and potentially infectious substances. This training should cover how to avoid inadvertent injury and mitigate the risk of transmitting blood borne pathogens.
DOT Regulations
The United States Department of Transportation (DOT) mandates training for employees packaging medical waste and offering it for shipping. Training should occur before the employee starts his or her job assignment as well as every three years thereafter.
Documentation Requirements
In addition to understanding what training requirements apply to your organization, it is also essential to have written verification that training occurred. This should include the date of training, name and qualifications of trainer, subject of training, and printed name, job title, and signature of the employee.
Your Protocol, Your Safety
Do your employees follow protocol for hospital waste disposal in your facility? Do you have a protocol? Secure Med can help with both. Our team can assist you in developing policies and procedures that will meet regulatory requirements and help you ensure that your staff is properly trained and everyone is safe.
Contact us today to schedule a consultation and evaluation.